Batteries are everywhere in day-to-day life – from smart phones to cars and come in many different sizes and chemistries, from small AA batteries in remote controls to the heavy lead-acid in internal combustion engine vehicles.
Lithium-ion batteries are rechargeable, light weight and energy dense batteries, you use them every day but have you thought:
Lets find out…..
All batteries are essentially two electrodes which are separated by an electrolyte.
A schematic of the materials and set-up of a Li-ion battery is shown below. The oxide electrode in this schematic is LiCoO2 – but other materials such as LiMn2O4 and LiNixMnyCozO2 (NMC) can be used. LiFePO4 is a polyanion alternative to these materials. The other electrode is graphite – have a think of where this material is in the classroom. Both of these materials are coated onto their respective current collectors. On charging, the lithium is removed from LiCoO2, transverses through the electrolyte and intercalates into the graphite. At the same time, an electron will move through the external circuit. On discharge the reverse process happens, but the electron will do work and hence charge our devices.
Learn more about charging / discharing cycle in a Li-ion battery by watching our Lithium Shuffle video
There’s a lot of analogies of battery manufacturing to how we bake a cake! Below the table tabulates the steps of making a cake, with the steps shown below and how this relates to the manufacture of a battery.
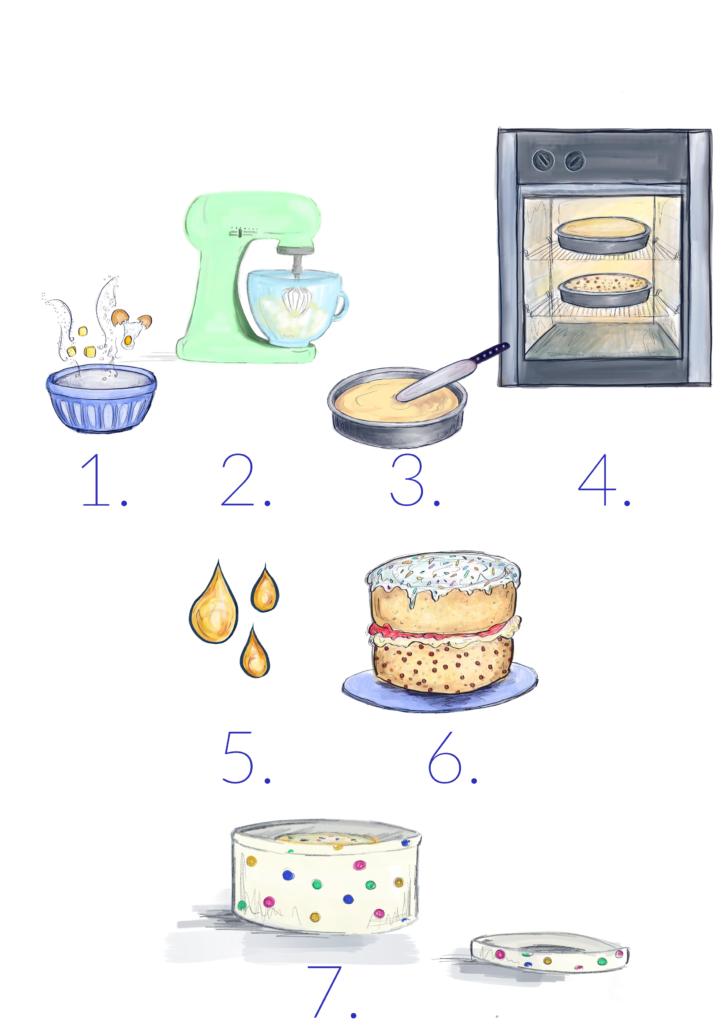
| Making a Cake | Making a Battery | |
| 1. |
We need to have all our ingredients ready to go – these need to be weighed. | Our electrode materials (such as LiCoO2) are synthesized and weighed.
A binder and a conductive additive needs to be available and weighed ready for mixing. |
| 2. |
All these ingredients have to be mixed to form a uniform mix. | All the materials are mixed together in a specific order, with the addition of solvent/water to form a slurry. |
| 3. |
The batter is then added to a cake tin. | The electrode ink is cast onto foil (aluminium or copper current collector). |
| 4. |
The mixture is then cooked. | The electrode ink is dried to remove the solvent. After this the electrodes are calendered – a step which compresses our layers down. You can visualise it as if you’re using a rolling pin to flatter you cake layer. |
| 5. |
The cake is then saturated with drizzle. | During cell manufacture, we have to use electrolyte for the conduction of the lithium ions between the electrodes. The electrolyte soaks into the electrodes. |
| 6. |
The cake is assembled. | The cell is assembled – there are many different types, here we’re representing a coin cell, as we have one electrode-electrolyte-second electrode set-up. |
| 7. |
And placed into a tin for safe transport. | To avoid any damage to the batteries, they need to be transported safely. |
Batteries have a finite lifetime and will with time degrade and their performance fade (learn more with our Battery Jenga activity)
While there is a growing demand for electric vehicles, forward thinking towards what happens to batteries at the end of their lifetime is required. Rather than sending these battery packs, which contain critical materials, to landfill – there are opportunities to recycle these cells!
Researchers in the Birmingham Battery Bunch, are focused on this challenge of recycling Li-ion batteries efficiently and effectively. The research covers several different stages of the recycling process, from shredding and physical processing, to electrolyte and binder extraction, chemical leaching and material re-synthesis.
During the shredding process, batteries are safely shredded in a custom-built facility designed to contain a battery fire. This then allows the shredded material to be separated by using their different properties such as magnetism, density or conductivities, before chemical treatments downstream to purify materials – have a look at the pictures below of a shredded Li-ion battery before and after separation.
*When we discharge a battery, the cathode will be the oxide electrode, as the Co4+ ions gain an electron and become Co3+. To maintain charge balance with the negative oxide ions, a lithium ion will intercalate (insert itself in the crystal lattice) – see Lithium Ion Shuffle video.
The video to accompany a recycling activity run during lockdown ( https://cotteridgepark.org.uk/scienceactivities/sciencebox7 )
Has downloadable infographics for the ‘Bake a Battery’ and other activites
The Faraday Institution’s project researcing new cathode materials