Written by: Anna Dickinson-Lomas
Differential Scanning Calorimetry, or DSC for short, is a technique used by scientists to understand how materials behave when heated or cooled. It is most commonly used for polymers (aka plastics).
There are two main things that might happen when a material is heated and/or cooled:
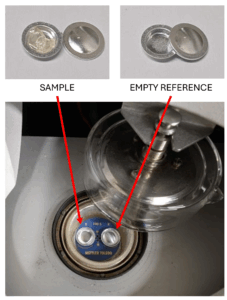
In DSC, a tiny amount of the material (~5 – 10 mg) is sealed in small metal dish. Another small metal dish is kept empty and used for comparison. The machine heats and cools both dishes and measures how much heat is taken in or given out by the sample.
This information is shown on a line graph, which will have peaks and troughs in the curve which represent any endothermic and exothermic reactions. This curve tells us about how the material changes behaviour, like when it melts or becomes harder.
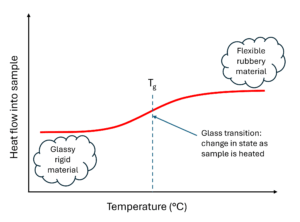
An important property of polymers or plastics is the glass transition temperature, which is when itchanges from flexible and rubbery to rigid and glassy, or the other way around. When you heat a hard plastic, you might notice it melts slowly, not instantly. This is because the change in state is a transition, not a sudden occurrence.
The change is caused by gradual movement in the polymer structure. When mobility in a rigid, glassy polymer is limited, the material can’t move so easily and is ‘stuck’ . When the material is heated its structure can become more mobile, so it can move around more freely. This is what we see when polymers melt.
This helps us to know what temperatures it is safe to use a certain material at (you don’t want to make something that gets hot out of something that softens and/melts at lower temperatures!).